

Pei Immunobio Coil Antigen Test Antigen Nasal Swab Rapid Diagnostic Test CE
Basic Info
| Model NO. | JSO001 |
| Type | IVD Reagent |
| Certification | CE/ISO13485/White List |
| Specimens | Serum, Plasma, or Whole Blood |
| Application | Neutralizing Antibody Test |
| Time | 10 Minutes Read Result |
| Delivery Time | 1 Week After Get Payment |
| Free Sample | Supply |
| Carton Weight | 13.5kg |
| Shelf Life | 18 Months |
| Carton Size | 64cm*44cm*39cm |
| Box Size | 18cm*12.3cm*7.8cm |
| Feature 1 | with Very High Accuarcy |
| Feature 2 | Fast Shipment |
| Feature 3 | High Quality But Best Price |
| Transport Package | Carton |
| Specification | 20 test/single test per box |
| Trademark | IMMUNOBIO |
| Origin | China |
| HS Code | 3002150050 |
| Production Capacity | 1 Million/Week |
Product Description
PEI/Bfarm Neutralizing Antibody Test Neutralizing AB test kit Neutralizing Antibodies Rapid Test
The Content of Nab Neutralizing Antibodies in the body is an important Indicator to Evaluation the effectiveness of VAC. The body of patients will produce anti-Vir antibodies a few days or a week after infected with the New Novel vir, Among them Among them, neutralizing antibodies are antibodies with anti-vir activity. Although they only account for a small part of the weight of anti-vir antibodies, neutralizing antibodies can Identify the vir surface protein, block the vir from binding to specific receptors on the cell surface, thereby preventing the vir from continuing to invade human cells

Features for Neutralizing AB Antibodies rapid test
A. Blood testing, Fingerstick whole blood is workable.B. Cutoff is 50ng/mLC. Easy to operate, no additional material required to run the assay
D. Little Specimen is required. 10ul of serum, plasma or 20ul of whole blood are enough.
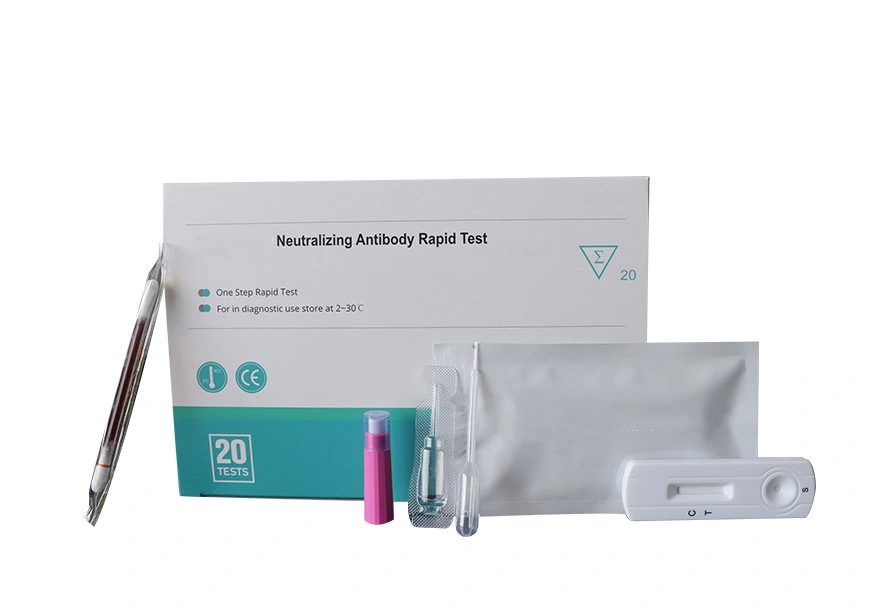
Authorized certifications for Neutralizing AB Antibodies rapid test
- CE Approved
- China's white list approved Neutralizing Antibody Rapid Test

Product Information for Neutralizing AB Antibodies rapid test
| Used For | Detection for Neutralizing antibodies in blood |
| Specimen | Serum, plasma, or whole blood |
| Certification | CE/ISO13485/White List |
| MOQ | 10000 tests |
| Delivery time | 1 week after Get payment |
| Packing | 20 test kits/Packing box 50 Boxes/Carton Carton Size:64*44*39cm |
| Test Data | Cut off 50ng/mL |
| Shelf Life | 18 months |
| Production Capacity | 1 Million/Week |
| Payment | Bank transfer |
Test producer for Neutralizing AB Antibodies rapid test
Allow the test device, specimen, buffer, and/or controls to equilibrate to room temperature (15-30°C) prior to testing.
1. Bring the pouch to room temperature before opening. Remove the test device from the sealed pouch and use it as soon as possible.
2. Place the test device on a clean and horizontal surface.
For Serum or Plasma Specimens:
Hold the dropper vertically, draw the specimen up to the Fill Line (approximately 10 μL), and transfer the specimen to the specimen well (S) of the test device, then add 3 drops of buffer (approximately 90 μL) and start the timer. See illustration below. Avoid trapping air bubbles in the specimen well (S).
For Whole Blood (Venipuncture/Fingerstick) Specimens:
To use a dropper: Hold the dropper vertically, draw the specimen 0.5-1 cm above the Fill Line, and transfer 2 drops of whole blood (approximately 20 μL) to the specimen well (S) of the test device, then add 3 drops of buffer (approximately 90 uL) and start the timer. See illustration below.
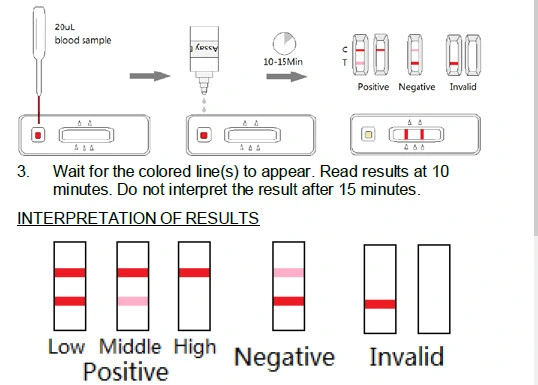
- Positive (+): Only C line appears, or T line is equal to C line or weaker than C line. It indicates that there are neutralizing antibodies in the specimen.
- Negative (-): Both the T line and C line appear, when the intensity of T line is stronger than C line. It indicates that there is no neutralizing antibodies in the specimen, or else the titer of neutralizing antibodies are of very low level.
- Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
Expected Results For Vac Reference.
The results if carrying vac are expected to be like below.
- Before first dose: Negative by rapid test
The results if carrying 19 vac are expected to be like below.
- Before first dose: Negative by rapid tes
3 weeks after first dose: weak or middle positive
- 1 week after second dose: middle or high positive
- 2 weeks after second dose: middle or high positiveCustomer Feedback: 5 droppers Whole blood+ 2 Dropper buffer
Company Profile
Hangzhou Immuno Biotech is a R&D based company located in Hangzhou. Immunobio is well known as a recombinant protein original designer and supplier in the upstream of in vitro diagnostic field. Immunobio is also a professional rapid test manufacturer who has advanced technology in veterinary diagnostic and human medical diagnostic industries. Immunobio has more than 30 authorized patents in IVD field and more than 20 under review.
To fight against the Vir , Immunobio has developed a series of rapid diagnostic test . In early February 2020, we released the IgG/IgM Antibody Rapid Test for the IgG and IgM antibodies testing. Then in September, Immunobio has successfully developed the Antigen Rapid Test to support the fast screening of antigen test. In December 2020, Neutralizing Antibody Rapid Test was developed in success, to indicate the protective status of the neutralizing antibodies in people's blood.
Hangzhou Immuno Biotech is doing and will continue doing innovative research and development in the IVD medical diagnostic field. Immunobio will keep our promise to provide the innovative and competitive products for a healthy world.
Certifcations
Logistics
Our Service
- Factory price, reasonable and competitive
- OEM/ODM service, Not only packaging and brand customization, we have our own laboratory and R&D team, which can develop and customize proprietary products for customers
- Timly and quickly feedback, anytime and anywhere for customer service.
- Provide professional training and quality samples to help customers develop the market
- Flexible trading methods to meet customer and business needs
More Rapid test kits
FAQ
Question 1: Can I get samples before play batch order?
A: Qulaified sample is available, details please contact with our sales.
Question 2: How about the MOQ?
A: MOQ is more than "0", but our sales team will advise you how much to order based on the products you need, national market conditions, international logistics costs and other factors
Question 3: How about the quality?
A: We already get CE, and also on the white list of Chinese goverment, furthermore, you can find our test result result on the wesite of German Ministry of Health
Question 4: Where is your factory? Can I visit your factory?
A:We are in Hangzhou, Alibaba also located in here, half an hour from Shanghai by high speed train. You are welcomed anytime that you visit us.
Question 5: Do you factory or trading company
A: We are factory, all product we supply is R&D and produce by ourself.
Question 6: How can I pay?
A: You can pay USD, EURO & RMB via T/T, PayPal or Western Union.
Question 7: Which certificate you have:
A: CE/ISO13485








